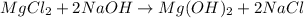We have several reaction-type options. At the end of the answer, I will update the image with the values that we are taking so as not to repeat them.
I will try to choose different ones to explain as much as I can:
Synthesis: It refers to when we have two reactants and form one from these two.
We can take:
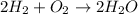
Decomposition: It is the opposite of synthesis, we have a reactant and it decomposes into more than one product
We can take:
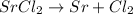
Simple displacement: An element or ion exchanges places with another.
We can take:
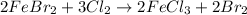
Double displacement: More than one molecule exchange places.
We can take: