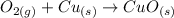Reaction 1
Solid sulfur can be represented by S(s) and solid iron is represented by Fe(s). Note the little s stands for solid.
Iron(III) sulfide will have the following formula:
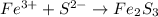
Reaction 2
Hyrogen gas can be represented by H2(g) and Iron (III) Oxide will have the following formula:
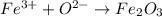
The products are H2O(l) and Fe(s). Therefore:
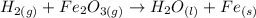
Reaction 3
Magnesium metal can be representd as Mg(s) and hydrochloric acid is HCl. Magnesium chloride will have the following formula:
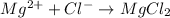
Therefore, the equation can be writen as:
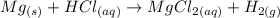
Reaction 4
Magnesium sulfide has the following formula:
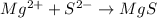
The reaction can be writen thus as:
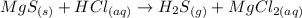
Reaction 5
Oxygen gas can be represented as O2(g) and solid copper as Cu(s). Copper (II) oxide has the following formula:
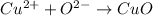
The reaction can be writen thus as: