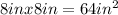SOLUTION:
Step 1:
Since the two figures are similar, it means that they have properties in common.
Step 2:
In the bigger figure, the side given is 10in and its area is 100 square inches meaning (10 in x 10 in).
Step 3:
In the smaller figure, the side given is 8in, and from step 1, we can see that the two figures are similar, so the area of the smaller figure will be (8 in x 8 in) = 64 square inches.
CONCLUSION:
The area of the smaller figure is 8in x 8in = 64 square inches