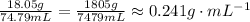To identify the mass of the solute, subtract the total mass by the mass of the graduated cylinder.
Thus, we have the following:
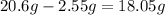
To obtain the concentration of the solution, divide the obtained mass by the total volume and then simplify.
Thus, we have the following: