Answer
47.60 g
Step-by-step explanation
Given that:
Molar mass of MgCl₂ = 95.20 g/mol
Mass of Mg produced = 12.15 grams
Equation: MgCl₂ → Mg + Cl₂
What to find:
To calculate the grams of MgCl₂ needed to produce 12.15 grams of Mg.
Step-by-step solution:
Step 1: Convert the mass of Mg produced to moles.
Using the molar mass of Mg (24.305 g/mol) and the mole formula, the moles of Mg produced is
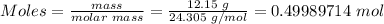
Step 2: Determine the moles of MgCl₂ needed to produce 12.15 g of Mg.
According to the given equation; 1 mol of MgCl₂ produced 1 mol of Mg
So, x mol of MgCl₂ will produce 0.49989714 mol of Mg
That is:
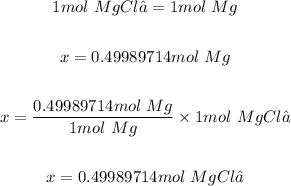
Step 3: Convert the moles of MgCl₂ needed to produce 12.15 g of Mg to grams.
Using the molar mass of MgCl₂ = 95.20 g/mol, therefore the mass in grams of MgCl₂ needed to produce 12.15 g of Mg will be:
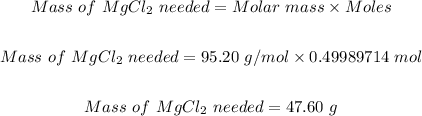
Hence, the grams of MgCl₂ needed to produce 12.15 grams of Mg is 47.60 g.