ANSWER
The oxidation state of nitrogen in NaNO2 is +3
Step-by-step explanation
Given information
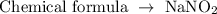
To find the oxidation state of nitrogen in NaNO2, follow the steps below
Step 1: Write NaNO2 in the ionic form
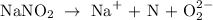
From the above reaction, you will see that the oxidation number of sodium is +1, and the oxidation number of oxygen is -2.
Step 2: Let the oxidation state of nitrogen be x
Add the oxidation numbers together and equate all to zero
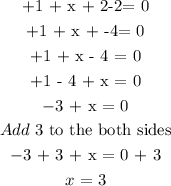
Hence, the oxidation state of nitrogen in NaNO2 is +3