This is a type of radioactive decay problem. Given an initial amount of N0, the amount at t years is given by
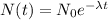
The information "27 years is the half-life of the substance" means that if we replace t by 27, we will get exactly half of the initial amount we had. That is
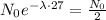
We can cancell out N0 on both sides, so we get
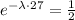
using the properties of exponentials, we have the equivalent equation
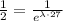
which is also equivalent to
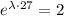
If we apply natural logarithm on both sides, we get
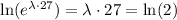
Finally, we can divide both sides by 27, to get
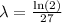
So, the function that describes the amount of the subtances at time t is given as
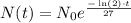
Now, we want to calculate the value of t, for which the amount we have at year t is exactly 66% of what we have at t=0. That is
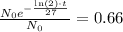
We can cancell out N0 and, by equivalence by using the properties of exponents, we get
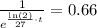
which is also equivalent to
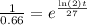
if we apply the natural logarithm on both sides, we get
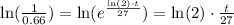
Finally, we want to solve for t. To do so, we can multiply by 27 and then divide by ln(2). So we get
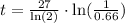
with help of a calculator, we have that t is approximately 16.185. That is, about after 16 years, we will have 66% of the initial amount.