Answer:
500,000cm²
Explanations:
The formula for calculating the perimeter of the fence is expressed as:
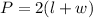
where:
• L is the ,length, of the fencing
,
• W is the ,width ,of the fencing
If Farmer Ed does not fence the side along the river, the perimeter of the river will become;
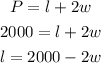
The area of the rectangular plot will be expressed as:
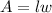
Substitute the expression for the length into the area to have:
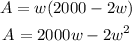
If the area of the plot is maximized, then dA/dw = 0. Taking the derivative will give:
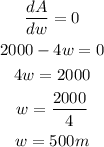
Calculate the length of the plot. Recall that:
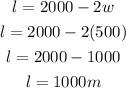
Determine the largest area that can be enclosed
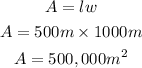
Hence the largest area that can be enclosed is 500,000cm²