Since we have that Hannah reads 3 pages every 8 minutes, we will construct the function by first knowing how many pages she reads in 1 minute, that is:
She reads 3/8 pages each minute, then we will construct the function as follows:
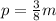
Where p is the number of pages and m is the number of minutes that she reads. We can see that this describes is if we solve for 1 minute and 8 minutes. If we solve for 1 minute, we get she read 3/8 pages and if we replace 8 minutes, we will get that she reads 3 pages.