First, we have to convert the number of molecules to the number of moles of CO2 (carbon dioxide), and to do this we can do the Avogadro's number:
The Avogadro's number helps us to determine the number of moles based on the number of molecules or atoms of a compound. This number is 6.022 x 10 ^(23) /mol.
So, the conversion from molecules to moles would be:
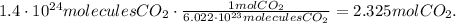
Now, using this data we can calculate the number of moles needed for propane (C3H8).
In the chemical reaction, you can see that 1 mol of propane produces 3 moles of CO2, so the calculation would be:
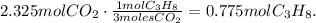
The answer is that 0.78 moles of C3H8 were burned and produced 2.325 moles of CO2 which is 1.4 x 10 ^(24) molecules of CO2.