The molarity of a solution is the molar concentration of it.
It is calculated by:
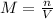
Where n is the number of moles of the substance in the solution and V is the total volume of the solution.
The volume was given:
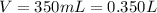
And the number of moles we can calculate using the given mass and the molar mass:
![\begin{gathered} m=4.67\operatorname{kg}=4670g \\ MM=34.6g/mol \end{gathered}]()
So:
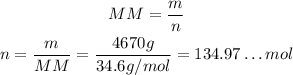
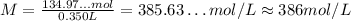
Thus, the molarity is approximately 386 mol/L.