For the reaction of Zn with HCl, the ratio is 1 mole of Zn to every 2 moles of HCl. So if there are 8.25 moles of Zn, it is possible to find the moles of HCl by doing the following relation:
1 mole Zn ----- 2 moles HCl
8.25 moles Zn ----- x moles HCl
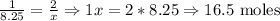
16.5 moles of HCl are needed to react with 8.25 moles of zinc.