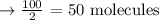Answer:
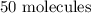
Step-by-step explanation:
Here, we want to know the number of molecules of oxygen that was reacted with
From the question, we can see that the mole ratio is 2 to 1
Thus, the number of molecules of oxygen reacted will be half that of hydrogen
Hence, the number of molecules of oxygen will be: