Let us first define the term molarity. Molarity is a way of expressing the concentration of a compound in solution and is defined as the number of moles per liter of solution.
We are given the molarity value, 1.00M, and the volume of the solution, 500 mL=0.5L. We can find the number of moles by clearing them from the following equation:
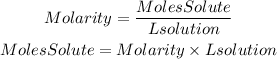
We replace the known data:
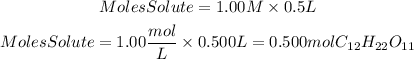
Now, the grams of sucralose are found by multiplying the moles found by the molar mass of sucralose, which is 342.3 g/mol:
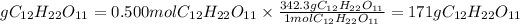
The quantity of sucrose required will be 171 g of sucrose