We have a change of pressure and temperature. To solve and find the new pressure we will assume that the volume and moles of the gas remain constant, therefore we can apply Gay-Lussac's law which tells us:
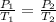
Where,
P1 is the initial pressure of the gas, 954atm
T1 is the temperature of the gas, 728K
T2 is the final temperature, 951K
P2 is the final pressure, =?
We clear P2 and replace the known data:
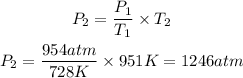
Answer: The new pressure will be 1246atm