The final pressure of the gas inside the ballon is 0.17 atm.
To solve this problem, we would need to use ideal gas equation.
Combined Gas Equation
This is a combination of the three major gas laws which are Boyle's law, Charles law and Pressure Law.
This is given as
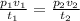
we can define our variables
V1 = 1.2L
P1 = 1.1 atm
T1 = 25°C = (25 + 273.15)K = 298.15K
V2 = 6.7L
T2 = 75°C = (75 + 273.15)K = 348.15K
P2 = ?
Let's substitute the values into the equation above and solve for the final pressure
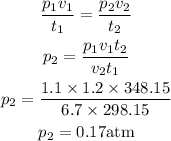
The final pressure of the gas inside the ballon is 0.17 atm.