Step 1 - Reading the chemical reaction
The given chemical reaction is:
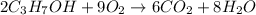
We can read this reaction as:
2 moles of C3H7OH react with 9 moles of O2 to produce 6 moles of CO2 and 8 moles of H2O
This is what we call theoretical reaction, i.e., a theoretical proportion that will be respected whenever this reaction happens.
As the exercise is specifically asking about the reactants, we can further simplify this statement to:
2 moles of C3H7OH react with 9 moles of O2
Step 2 - Converting this proportion to grams
We can convert moles to grams by multiplying the number of moles by the molar mass of each substance (32 g/mol for O2; 60 g/mol for C3H7OH):
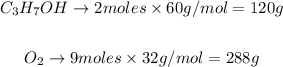
Therefore, we obtain the following relation:
288g of O2 react with 120g of C3H7OH
This is a fixed proportion. Whenever this reaction happens, it will respect this proportion. We can use it thus to find the limiting reactant.
Step 3 - Finding the limiting reactant
To discover the limiting reactant, we first must discover how much O2 is needed to completely react with 8 grams of C3H7OH:
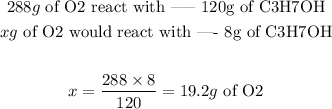
Therefore, in order to completely consume 8 grams of C3H7OH, we would need 19.2g of O2. Since there less O2 than needed, it's the limiting reactant.
Answer: C3H7OH is in excess. Therefore, O2 is the limiting reactant.