Answer:
The new volume of the balloon is 294 cm^3.
Step-by-step explanation:
The given information from the exercise is:
- Initial volume (V1): 641 cm^3
- Initial temperature (T1): 435 K
- Final temperature (T2): 200 K
To calculate the final volume (V2) of the balloon, we can use the Charles's law formula, by replacing the values of V1, T1 and T2:
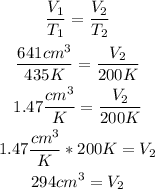
So, the new volume of the balloon is 294 cm^3.