1) List the quantities.
Sample: 700 g Ni3(PO3)2
2) Convert gramas of Ni3(PO3)2 to moles of Ni3(PO3)2.
The molar mass of Ni3(PO3)2 is 334.0241 g/mol.
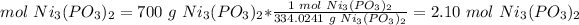
3) Convert moles to particles.
1 mol = 6.022*10^23 particles.
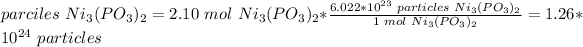
700 g Ni3(PO3)2 is equal to 1.26*10^24 particles.