Chemistry => Thermochemistry => Enthalpy
We have a material that is heated until it goes from a solid state to a liquid state, that is, it melts.
To calculate the total energy we will take into account two stages:
1. Corresponds to the energy needed to raise the temperature from 20°C to 660°C.
2. The energy needed to melt aluminum at a constant melting temperature (660°C).
We will need the following data that we can find in the literature:
Specific heat of aluminum = 0.896J/g°C
enthalpy of fusion aluminum = 0.322J/g
For stage 1. the energy required will be calculated with the following equation:
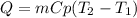
Where,
Q is the energy required to raise the temperature
m is the mass of the aluminum soda can, 14g
Cp is the specific heat of aluminum, 0.896J/g°C
T2 is the final temperature, 660°C
T1 is the initial temperature, 20°C
We replace the known data:
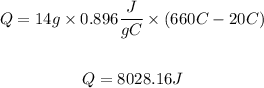
For stage 2 the energy required will be calculated with the following equation:
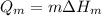
Where
Qm is the energy required to melt the aluminum soda can
m is the mass, 14g
deltaHm is the enthalpy of fusion aluminum,0.322J/g
The energy required will be:
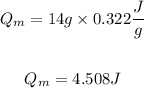
So, the total energy will be the sum of both energies. So, we will have:
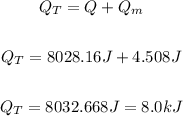
Answer: It would take 8.0 kJ to melt 1 aluminum soda can