Answer:
0.508 moles
Step-by-step explanation:
In order to find the number of moles the gas with the above given variables we use the formula
PV = nRT
where
p is the pressure
v is the volume of the gas
n is the number of moles
T is the absolute temperature of the gas
R is molar gas constant
Here since the pressure is in atm and the volume in litres the molar gas constant will be
0.082057 L·atm·K-¹·mol-¹
From the question
p = 0.5 atm
v = 25 L
T = 300 K
Since we're finding the number of moles we make n the subject
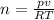
We have
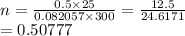
We have the final answer as
0.508 moles
Hope this helps you