149.0g of zinc. The mass of zinc used in the single-replacement reaction of copper sulfate and zinc is 149.0 g.
The balanced chemical equation for the single-replacement reaction of copper sulfate and zinc is:
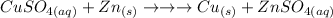
This reaction produces zinc sulfate, but the concentration of the solution is unknown. To determine the mass of zinc used, we can use the following steps:
Calculate the moles of copper produced from the mass of copper produced.
Calculate the moles of zinc used from the moles of copper produced using the stoichiometric ratio of the balanced chemical equation.
Convert the moles of zinc used to grams of zinc using the molar mass of zinc.
Step 1: Calculate the moles of copper produced from the mass of copper produced.
Moles of copper produced = Mass of copper produced / Molar mass of copper
Moles of copper produced = 145.0 g / 63.55 g/mol
Moles of copper produced = 2.281 mol
Step 2: Calculate the moles of zinc used from the moles of copper produced using the stoichiometric ratio of the balanced chemical equation.
The balanced chemical equation tells us that 1 mole of copper sulfate reacts with 1 mole of zinc to produce 1 mole of copper and 1 mole of zinc sulfate. Therefore, the moles of zinc used is equal to the moles of copper produced.
Moles of zinc used = Moles of copper produced
Moles of zinc used = 2.281 mol
Step 3: Convert the moles of zinc used to grams of zinc using the molar mass of zinc.
Grams of zinc used = Moles of zinc used * Molar mass of zinc
Grams of zinc used = 2.281 mol * 65.38 g/mol
Grams of zinc used = 149.0 g
Therefore, 149.0 g of zinc was used in the single-replacement reaction of copper sulfate and zinc.