The first step to solve this question is to convert the given amount of grams of Fe to moles using its molecular weight.
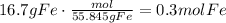
According to the given reaction, 4 moles of Fe produce 2 moles of Fe2O3. Use this ratio to find how many moles of Fe2O3 are formed:
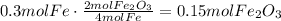
Convert this amount of moles to grams using Fe2O3 molar mass:
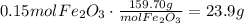
It means that 23.9 grams of Fe2O3 are formed.