Answer
The partial pressure of oxygen in atm = 0.472 atm
Step-by-step explanation
Dalton’s Law of Partial Pressure states that the total pressure exerted by a mixture of gases is equal to the sum of the partial pressure of the individual gases present in the container.
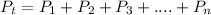
Given:
The total pressure, Pt = 725 mmHg,
the partial pressure of nitrogen, P₁ = 148 mmHg, and
the partial pressure of argon, P₂ = 218 mmHg
What to find:
The partial pressure of oxygen, P₃ in atm.
Step-by-step solution:
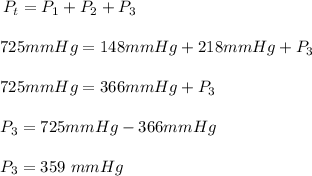
The final step is to convert the partial pressure of 359 mmHg oxygen to atm.
So 359 mmHg will be (359 mmHg x 1 atm)/(760 mmHg) = 0.472 atm
Hence, the partial pressure of oxygen in atm = 0.472 atm