SOLUTION
From the question, we have been given the equation for pH and we want to calculate the concentration of hydrogen given as H+
This becomes
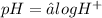
We were given the pH as 4. Substituting this into the equation, we have
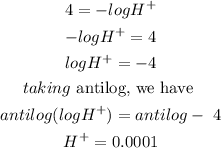
Hence the answer is 0.0001 the last option