Answer:
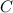
Step-by-step explanation:
Here, we want to calculate the percentage composition of the compound formed when oxygen reacts with iron
We have the equation of reaction as follows:
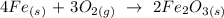
The compound formed is Fe2O3
Now, let us get its percentage composition
The molar mass of the compound is 160 g/mol
The atomic mass of iron is 56 amu
The atomic mass of oxygen is 16 amu
Now, let us get the percentage composition:
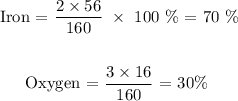
The closest here is thus the option C