ANSWER
The final volume of the balloon is 12.67L
Step-by-step explanation
Given that;
The initial volume of the ballon is 12dm^3
The initial temperature at STP is 273.15 degrees Celcius
The initial pressure at STP is 760 mmHg
The final temperature is 0 degrees Celcius
The final volume is 720 mmHg
Follow the steps below to find the new volume of the balloon
Step1; Write the general gas law equation
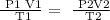
Step 2; Convert the volume and temperature to liters and degrees Kelvin

Step 3; Substitute the given data into the formula above to find the final volume
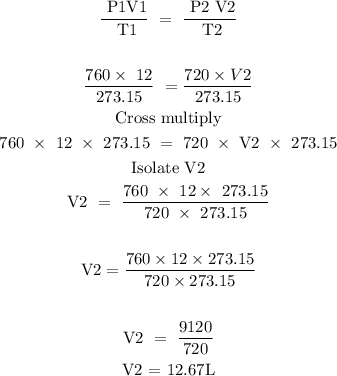
Hence, the final volume of the balloon is 12.67L