The formula we are going to use is:
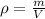
Where
p is density, in g/mL
m is mass, in grams
V is volume, in mL or cm^3
Density is given and we have to find the volume.
The gold bar is in a shape of rectangular prism, so we need to multiply the width, height, and length (given) of the gold bar to find its volume. Let's do it:
![\begin{gathered} V=1.2*1.6*23 \\ V=44.16\operatorname{cm}^3 \end{gathered}]()
Now, we substitute and solve for m, the mass (weight) in grams:
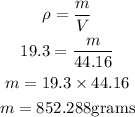
The mass is abut 852.29 grams