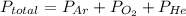Answer
C.
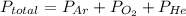
Step-by-step explanation
If argon (Ar), oxygen (O), and helium gas (He) are placed in a container together, they would not react with each other due to the unreactivity property of the noble gases (Ar, and He).
So, According to Dalton's law of partial, the total pressure exerted by a mixture of gases that do not react chemically is equal to the sum of their partial pressure.
Hence, the option that correctly gives the expression to compute the total pressure of the gases is C.