INFORMATION:
We need to use Lewis symbols for PH3 and, we must point out bonding pairs and lone pairs.
STEP BY STEP EXPLANATION:
We know that the Electron dot structures and Lewis dot formula are the same. We can draw it if we know the molecular formula of the compound. It is also useful to represent the atomic state of elements.
In this case, the molecular formula is
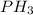
Then, we need to calculate the total number of valence electrons
As we know that, phosphorous has '5' valence electrons and hydrogen has '1' valence electron. Then, the total number will be
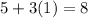
So, the total number of valence electrons for PH3 is 8
Now, from molecular formula we know that there are one atom of P and 3 of H.
To do the Lewis dot formula, we need to put the P in the center and H always go on the outside.
Finally, we will put 2 electrons between each of the atoms to for chemical bonds. Since we have three H, we will use 6 valence electrons, so we need to put the last two electrons in the center for a total of 8 valence electrons.
The Electron dot structure would be
Where,
- green represents bonding pairs
- red represents lone pairs
ANSWER:
Where,
- green represents bonding pairs
- red represents lone pairs