The energy of the X-ray is,
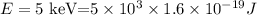
the wavelength of the X-ray is then,
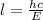
Here,
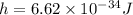
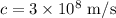
substituting the values we get the wavelength,
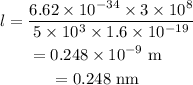
we can see here the given atomic space is nearly equal to the order of the calculated wavelength
for the Bragg's law experiment this is the main condition that the atomic spacing should be of the order of the wavelength of the light
Hence the given X-ray can be used for the purpose.