Answer:
94.4 %.
Step-by-step explanation:
First, let's see the chemical equation:
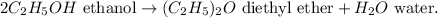
Now, let's calculate the number of moles of ethanol of 517 g of ethanol using its molar mass, which is 46 g/mol, like this:
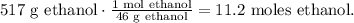
Now, let's see how many moles of diethyl ether can be produced by 11.2 moles of ethanol. You can see in the chemical equation that 2 moles of ethanol reacted produces 1 mol of diethyl ether:
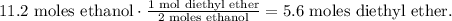
Now, let's convert 5.6 moles of diethyl ether to grams using the molar mass of diethyl ether which is 74 g/mol:
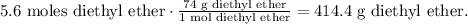
414 g of diethyl ether would be the theoretical yield.
The final step is to calculate the percent yield using the following formula:
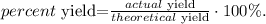
The actual yield of diethyl ether is the given value of the experiment, which is 391 g. If we replace the data that we have, we obtain:
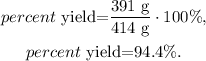
The percent yield of this experiment is 94.4 %.