Answer:
The following equations below are redox reactions:
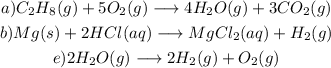
Explanations:
Redox reaction occurs when there is the addition of oxygen and the removal of hydrogen in a chemical reaction. Redox is a type of chemical reaction in which the oxidation states of atoms are changed.
According to the question, we are to determine which of the equation listed undergoes a redox reaction.
a) For the equation
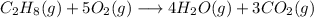
Propane compound C3H8 gains oxygen and loses hydrogen simultaneously to form CO2, Therefore the reaction is a redox
b) For the reaction:

From this equation, you can see that Magnesium metal is being oxidized to magnesium cations, Mg2+. while hydrogen is being reduced from hydrogen ions, H+, to produce hydrogen gas, H2. Hence we can conclude that the reaction is also a redox reaction.
c) For the reaction:
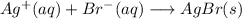
from the reaction, you can see that the oxidation state of silver in Ag+ and AgBr is +1 and since there is no change in oxidation state, hence the reaction is NOT a redox reaction
d) For the reaction:
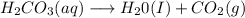
This is not a redox reaction since the formation of water and carbon dioxide itself undergo a redox reaction.
e) For the chemical reaction
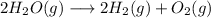
Hydrogen is known to oxidize to H+ ion while oxygen is being reduced O2-. Hence we can conclude that it is a redox reaction.
f) For the chemical reaction:

This is not a redox reaction as well since there is no change in the oxidation number of the elements.