Starting with the equation:
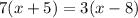
Use the distributive property to expand both parenthesis:
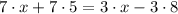
Solve the products 7*5 and 3*8:
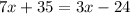
Substract 35 from both sides of the equation:
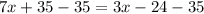
Solve the additions 35-35 and -24-35:
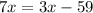
Substract 3x from both sides of the equation:
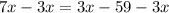
Add like terms in both sides of the equation:
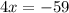
Divide both sides of the equation by 4:
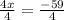
Simplify the fraction 4/4:
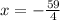
Check this answer by plugging in the value of x in the original equation:
![undefined]()