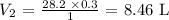Answer:
Step-by-step explanation:
According to Boyles' law, the volume of a given mass of gas is inversely proportional to its pressure
Mathematically:
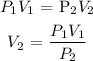
Where:
P1 is the initial pressure which is 28.2 atm
V1 is the volume of the cylinder which is:
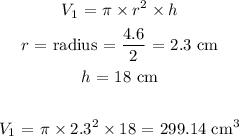
We convert V1 to liters by multiplying by 0.001 L
That would be:
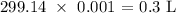
P2 is the atmospheric pressure which is 1 atm
Substituting the values, we have: