Answer:
200 g.
Step-by-step explanation:
What is given?
Initial quantity (N₀) = 400 g.
Half-life of rubidium-87 = 4.8 x 10¹⁰ years.
Step-by-step solution:
Let's see the formula to calculate the quantity remaining:
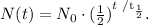
Where N₀ is the initial quantity, t is the time, and t (1/2) is the half-life of the substance, in this case, Rb-87. Based on this, our formula will be:
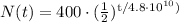
If we want to find the amount of Rb-87 after 1 half-life, our t would be equal to 4.8 x 10¹⁰ years, so replacing this value in the formula we obtain:
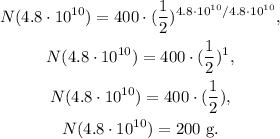
The answer is that after 1 half-life of a 400 g sample of Rb-87, the remaining quantity is 200 g.