ANSWER
The mass of Ca(NO3)2 is 19.692 grams
Explanation:
Given information
The mass of calcium hydroxide = 8.90g
Step 1: Write a balanced equation for the reaction

From the above reaction, you will see that 1 mole of calcium hydroxide reacts with 2 moles of nitric acid to give one mole of calcium nitrate.
Step 2: Find the number of moles of calcium hydroxide using the below formula
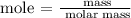
According to the periodic table, the molar mass of calcium hydroxide is 74.093 g/mol
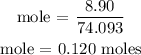
Step 3: Find the number of moles of calcium nitrate using a stoichiometry ratio
From the above reaction, you will see that 1 mole of calcium hydroxide reacts with 2 moles of nitric acid to give 1 mole of calcium nitrate.
Let x represents the number of moles of Ca(No3)2
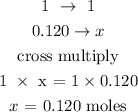
Hence, the number of moles of Ca(NO3)2 is 0.120 moles
Step 4: Find the mass of ca(NO3)2 using the below formula
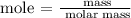
According to the periodic table, the molar mass of Ca(NO3)2 is 164.1 g/mol
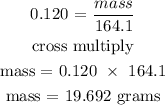
Hence, the mass of Ca(NO3)2 is 19.692 grams