Answer and explanation:
1st) It is necessary to write the products of the reaction. We can write the products combining the positive parte of CaCO3 (Ca+2) with the negative part of BPO3 (PO3-3) with the corresponding subscripts, so one product will be Ca3(PO3)2.
In the same way, we can obtain the second product, combining the negative part of CaCO3 (CO3-2) with the positive part of BPO3 (B+3), so the other product will be B2(CO3)3.
2nd) Now we can write and balance the chemical equation:
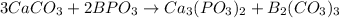
The equation is balanced, because in each side of the reaction there are: 3 Ca, 3 C, 15 O, 2 B and 2 P.