Answer:
The mass of water is 25.9g.
Step-by-step explanation:
1st) It is necessary to use the Heat formula. Knowing that the piece of aluminum releases heat and the water absorbs heat, the equation is a sum of both heats euqal to zero:
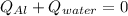
2nd) The information given in the exercise is:
• Piece of aluminum:
- Mass (mAl)=55.0g
- Heat capacity (cAl)=0.902J/°C*g
- Initial Temperature (TiAl)= 72.4°C
- Final Temperature (TfAl)= 44.9°C
• Water:
- Mass (mwater)= this is what we have to calculate.
- Heat capacity (cwater)= 4.18J/°C*g
- Initial Temperature (Tiwater)= 32.3°C
- Final Temperature (Tfwater)= 44.9°C
3rd) It is necessary to replace the values in the formula to calculate the mass of the aluminum piece:
![\begin{gathered} -Q_(Al)=Q_(water) \\ -\lbrack m_(Al)*c_(Al)*(T_(fAl)-T_(iAl))\rbrack=m_(water)*c_(water)*\left(T_(fwater)-T_(iwater)\right) \\ -\lbrack55.0g*0.902(J)/(\degree C*g)*(44.9°C-72.4°C)\rbrack=m_(water)*4.18\frac{J}{\operatorname{\degree}C*g}*(44.9°C-32.3°C) \\ -\lbrack-1364.28J\rbrack=m_(water)*52.67\frac{J}{\operatorname{\degree}C} \\ (1364.28J)/(52.67(J)/(g))=m_(water) \\ m_(water)=25.9g \\ \end{gathered}]()
Resolution number 2 (image):
Finally, the mass of water is 25.9g.