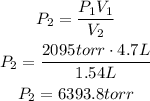To solve this problem we have to apply Boyle's Law, that states that the product of the pressure and the volume of a system of ideal gases is a constant, this can be expressed as:
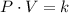
When there is a change in the system, this Law can be expressed as:
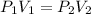
In this case, P1 has a value of 2095torr, V1 a value of 4.7L and V2 a value of 1.54L, use these values to find P2: