Answer
The mass of the product, PbI₂ (s), the student should expect is 2.784 grams.
Step-by-step explanation
Given:
Mass of lead (II) nitrate = 2 g
Mass of potassium iodide = 4 g
Using the atomic masses elements in the periodic table;
Molar mass of lead (II) nitrate (Pb(NO₃)₂) = 331.2 g/mol
potassium iodide (KI) = 166.0028 g/mol
What to find:
The product in grams that is produced.
Step-by-step solution:
Step 1: Write a balanced chemical equation for the reaction.
Pb(NO₃)₂ (aq) + 2KI (aq) → 2KNO₃ (aq) + PbI₂ (s)
Step 2: Convert the given masses to moles
Using the mole formula
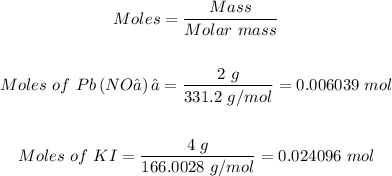
Step 3: Determine the limiting reactant.
There are more moles of potassium iodide than of lead (II) nitrate (from step 2), but the ratio is only the following:
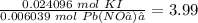
Because the ratio of the coefficients in the balanced chemical equation is,
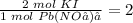
There is not enough lead (II) nitrate (Pb(NO₃)₂) to react with all the potassium iodide (KI), thus, lead (II) nitrate (Pb(NO₃)₂) is the limiting reactant that determines the grams of the product produced.
Step 4: Calculate the moles of the product ( PbI₂) produced using the mole ratio in step 1 and the number of moles of Pb(NO₃)₂ in step 2.
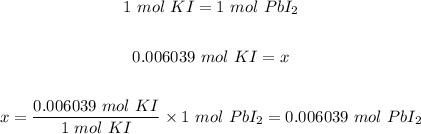
Step 5: Convert 0.006039 mol PbI₂ to grams using the same formula in step 2.
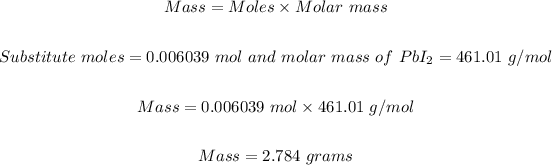
Therefore the mass of the product, PbI₂ (s), the student should expect is 2.784 grams.