Given information:
Density: 5.67 g/mL
Volume: 20 mL
Chemistry -> Measurements -> Density
Based on the density of a compound, we can make conversions between solid and liquid states. Let's take a look at the following relation:
5.67 g -------- 1 mL
x g --------- 20 mL
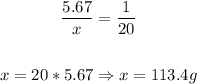
To have a volume of 20 mL, 113.4 grams of this compound are needed.