Solution:
The numbers given are directed numbers.
Directed numbers are integers that have a particular size/magnitude (positive or negative).
To get the product of two directed numbers, the sign is also taken into consideration.
Below is the result got from the product of the signs of directed numbers.

Hence, from the above;
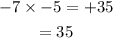
Therefore, the product of -7 and -5 is 35.