ANSWER
The density of the block is 4.5 g/cm^3
The block is identified as titanium
Physical properties
1. Titanium has a low density
2. Titanium has a very high strength
Chemical properties
1. It is radioactive in nature
Step-by-step explanation
Given that;
The length of the block is 3.00 cm
The height of the block is 5.00 cm
The width of the block is 5.00 cm
The mass of the block is 337.50g
Follow the steps below to find the density of the block
Step 1; Find the volume of the block
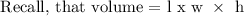
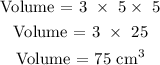
The volume of the block is 75 cubic meters
Step 2; Find the density of the block using the formula below
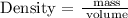
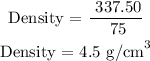
The density of the block is 4.5 g/cm^3
Hence, the block is identified as titanium
The next step is to write the physical and chemical properties of titanium
Physical properties
1. Titanium has a low density
2. Titanium has a very high strength
Chemical properties
1. It is radioactive in nature