Remember that the formula of half-life is the following:
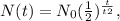
Where N(t) is the amount in grams, N(0) is the initial amount and t2 is the half-life in seconds.
Replacing the data that we have, which N(0) is 60 g and t2 is 8 seconds, we're going to obtain:
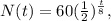
And the problem is asking for the amount of Au-180 in grams after 32 seconds, so t would be 32:
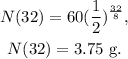
The answer is that after 32 seconds, it will be 3.75 grams of Au-180.