The equation that relates temperature change and heat is as follows:
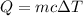
Where Q is the heat, m is the mass, c is the specific heat capacity and ΔT is the change in temperature (final temperature minus initial temperature.
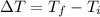
The specific heat capacity is given:
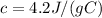
And the mass is also given:
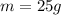
And the initial temperature is 30°C and the final is 33°C, so:
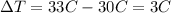
So, putting altogether, we have:
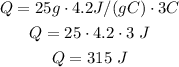
So, we need 315 J of energy.