Answer:
204 grams
Explanations:
The chemical reaction formed from the reaction of Nitrogen and hydrogen is expressed as:
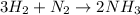
From the given reaction based on stochiometry, you can see that 1 mole of nitrogen produces 2 moles of ammonia.
Therefore, 6 moles of nitrogen will produce 12 moles of NH₃
Calculate the mass of ammonia produced
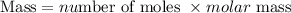
The molar mass of NH₃ = 14 + (1 * 3) = 17g/mol
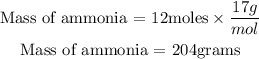
Therefore, the mass of ammonia (NH3) produced from 6 moles of nitrogen is 204 grams.