To determine the freezing point we can apply the following formula that relates the molality to the freezing point of water:
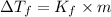
Where,
m is the molality
Kf is the freezing point of water
DeltaTf= Freezing point
We replace the known data:
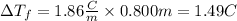
So, the freezing point of the solution will be 1.49°C