The balanced equation of the described combustion reaction is:
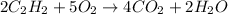
We see that for every 2 moles of acetylene 5 moles of oxygen are required, so first, we must calculate the moles of acetylene that correspond to 32.5 grams, for this we use the molar mass of acetylene equal to 26.04g/mol.
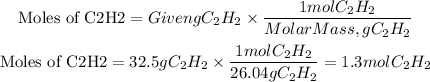
Now, we have that the mole ratio between C2H2 and O2 is 2 to 5. Therefore, the moles of O2 needed will be:
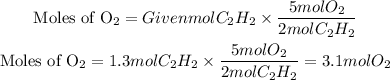
Now, the grams of oxygen will be:
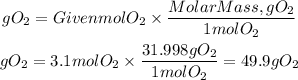
To combust 32.5g of C2H2 are required 49.9 g of O2