Answer:
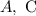
Step-by-step explanation:
Here, we want to select the options that are correct when describing ionic bonds
In an ionic bond, we have an atom with excess electrons (the metal atom) donating its excess electrons to the non-metallic atom (the electron-deficient atom)
Thus, one atom gives, while the other take
This makes the first option correct
When the metal donates the electron, it becomes positively charged (cation). When the non-metal receives the electron, it becomes negatively charged (anion)
This makes the third option correct